-
 Overview
Overview
Centre Testing International Group Co., Ltd. (NG28) is a market leader in testing, inspection, certification, calibration, audit, training & technical services; building trust between governments, enterprises, and consumers.
-
 Sustainability
SustainabilitySustainability is deeply rooted in NG28’s business model, by delivering science-based solutions and verification services, to increase transparency and traceability throughout the global value chain. NG28 is a proponent of carbon neutrality and sustainable development.
-
 Our service
Our serviceCentre Testing International Co., Ltd. (NG28) is the pioneer and leader in the TIC Industry which provides one-stop solutions on testing, inspection, certification, calibration, audit, training & technical services.
-
By Industry
Our service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
 Environment
Environment
-
 Raw Material & Fuel Chemicals
Raw Material & Fuel Chemicals
-
 Textiles, Apparel, Footwear & Accessories
Textiles, Apparel, Footwear & Accessories
-
 Food & Agricultural Products
Food & Agricultural Products
-
 Cosmetics, Personal Care & Household Chemicals
Cosmetics, Personal Care & Household Chemicals
-
 Building Materials&Construction Engineering
Building Materials&Construction Engineering
-
 Electronic & Electrical Appliances
Electronic & Electrical Appliances
-
 Toys, Furniture & Home Decoration
Toys, Furniture & Home Decoration
-
 Industrial Equipment & Manufacturing
Industrial Equipment & Manufacturing
-
 Rail & Aviation
Rail & Aviation
-
 Automotive & Spare Parts
Automotive & Spare Parts
-
 Pharma and Medical Services
Pharma and Medical Services
-
 Maritime Vessel Compliance Testing
Maritime Vessel Compliance Testing
 By Industry
By IndustryOur service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
-
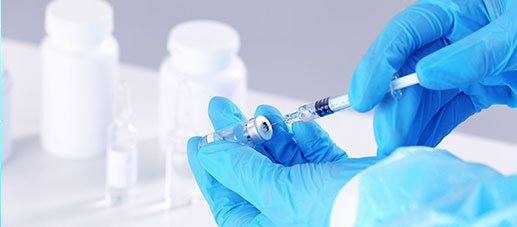 Specialty
SpecialtyComprehensively guarantee quality and safety, promote compliance and innovation, demonstrate brand competitiveness, and achieve higher quality, healthier, safer, and greener sustainable development.
-
 Management
ManagementWe have established a clear governance structure in accordance with listing requirements and national regulations and policies to deal with internal and external challenges and achieve sustainable development.
-
 Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
-
 Talents Policy
Talents PolicyEnsuring the basic rights and benefits of employees;
Providing professional skills training to promote employees’ growth;
Carrying out various kinds of activities to balance employees’ work and life.
-
 RecruitmentWelcome to join NG28 family! We are providing a platform for you to show your talents and achieve your career aspiration.
RecruitmentWelcome to join NG28 family! We are providing a platform for you to show your talents and achieve your career aspiration.

QUALITY & VALUE
Microbiological testing is an indispensable part of pharmaceutical quality assurance, and NG28 China Testing has a GMP-compliant microbiology laboratory with industry-leading equipment and facilities and a team of professional technicians. We can provide microbial limit check, sterility check, bioburden, culture media quality control test, bacterial endotoxin, disinfectant validation, package sealing study (microbial challenge method) and other tests that meet the requirements of GMP, ISO and other systems.

Microbial Limit Check
Microbial limit is used to check the degree of microbial contamination of non-sterile products, their raw and auxiliary materials, and pharmaceutical packaging materials, and whether the limit requirements are met.
NG28 provides method suitability (method validation) and testing services for total aerobic count, total mold and yeast count, and control organisms check according to the requirements of the Chinese Pharmacopoeia, the U.S. Pharmacopoeia, the European Pharmacopoeia, and other national pharmacopoeias.
Sterility Test
Sterility testing is a method used to check the sterility of sterile drugs, biological products, medical devices, raw materials, excipients and other species.
NG28 offers method suitability and testing services for sterility testing in accordance with the pharmacopoeias of various countries.
 |
 |
Bioburden
Bioburden refers to the total number of microorganisms surviving on medical devices, semi-finished products and packaging. Bioburden is used to assess the degree of microbial contamination of products before sterilization.
NG28 can develop test programs according to the needs of product characteristics and provide method applicability and testing services for bioburden.
Bacterial Endotoxin Test
Gram-negative bacteria can release endotoxin after death or autolysis, which can act on the thermoregulatory center to cause a feverish response. Bacterial endotoxin assay is used to detect the presence of this type of toxin in samples.
NG28 provides method validation and testing services for endotoxin gel method, dynamic turbidimetric method and dynamic colorimetric method.

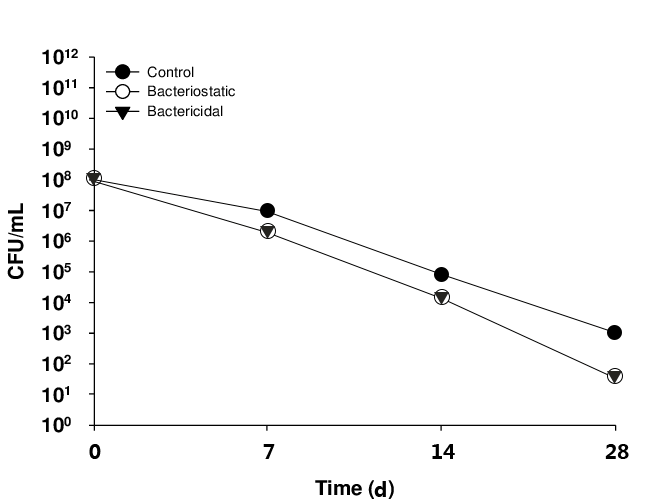
Bacteriostatic Efficacy ChecK
Bacteriostatic agents are substances that inhibit the growth of microorganisms. Bacteriostatic potency testing is used to determine the bacteriostatic ability of sterile and non-sterile preparations, and is used to guide the determination of the type and concentration of bacteriostatic agents in the preparation during the R&D stage of the product.
NG28 provides bacteriostatic potency test neutralizer validation and testing services.
Culture Medium Quality Control Test
Culture medium is the basis of microbiological test, which directly affects the results of microbiological test, and the quality of culture medium can be assessed whether it meets the requirements of relevant regulations through the quality control test of culture medium.
NG28 provides testing services for culture medium pH value, suitability check and sensitivity check.
|
Culture medium pH value |
Suitability check |
Sensitivity Check |
Package Seal Study (Microbial Challenge Method)
The sealing integrity of sterile pharmaceutical packaging containers is an important quality attribute of the pharmaceutical packaging system, and its quality monitoring runs through the entire life cycle of pharmaceutical production.
NG28 has research experience in a variety of rigid and flexible packaging materials, including glass ampoules, glass infusion bottles, polypropylene infusion bottles, non-PVC soft bags, prefilled injections, etc. We provide microbial challenge testing services and can design complete research plans, complete research experiments, and conduct correlation analysis of the results according to different product needs.
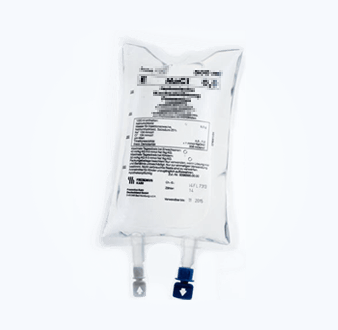 |
 |
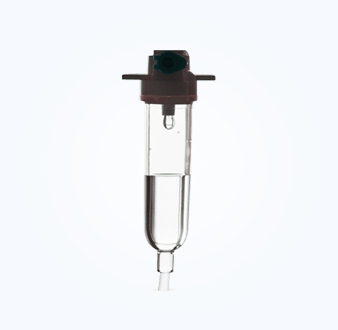 |
|
Disinfectant Validation
Disinfectant is a medium used to kill microorganisms. It is used for daily cleaning of clean areas and is an important means to ensure a clean environment. Disinfectant verification is used to check the ability of disinfectants to kill microorganisms on carrier surfaces (such as stainless steel, color steel plates, PVC, etc.) and to confirm the disinfection effectiveness of disinfectants.
NG28 provides testing services for disinfectant quantitative killing experiments, carrier surface disinfection effect verification, and disinfectant validity period verification. Disinfectant verification solutions can be customized according to customer needs.
|
Quantitative Killing Experiment |
Carrier Surface Disinfection Effect |
Disinfectant Validity Verification |
Solution Customization |
Microbiological Testing
he evaluation of microorganisms on medical devices, semi-finished products or packaging includes two aspects: the evaluation of the number of viable microorganisms and the evaluation of microbial characterization. Microbial characterization is necessary to discover changes in the microbiome of the product, which may affect the establishment of the sterilization process. Microbial testing includes aspects such as colony cell morphology, population morphology, biochemical properties and gene sequence.
NG28 provides testing services such as microbial morphology, biochemical testing and DNA feature sequence testing.
|
Microbial Morphology |
Biochemical Testing |
DNA Sequence Analysis |
- About NG28
- Our Services
- Investor Relations
- NG28 Mall
-
Resource Center
- Application Forms
- Bulletin
- Training Center
- NG28 Academy
- Reports Validation
-
Join Us
- Talents Policy
- Recruitment





























 粤公网安备 44030602000441号
粤公网安备 44030602000441号 