-
 Overview
Overview
Centre Testing International Group Co., Ltd. (NG28) is a market leader in testing, inspection, certification, calibration, audit, training & technical services; building trust between governments, enterprises, and consumers.
-
 Sustainability
SustainabilitySustainability is deeply rooted in NG28’s business model, by delivering science-based solutions and verification services, to increase transparency and traceability throughout the global value chain. NG28 is a proponent of carbon neutrality and sustainable development.
-
 Our service
Our serviceCentre Testing International Co., Ltd. (NG28) is the pioneer and leader in the TIC Industry which provides one-stop solutions on testing, inspection, certification, calibration, audit, training & technical services.
-
By Industry
Our service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
 Environment
Environment
-
 Raw Material & Fuel Chemicals
Raw Material & Fuel Chemicals
-
 Textiles, Apparel, Footwear & Accessories
Textiles, Apparel, Footwear & Accessories
-
 Food & Agricultural Products
Food & Agricultural Products
-
 Cosmetics, Personal Care & Household Chemicals
Cosmetics, Personal Care & Household Chemicals
-
 Building Materials&Construction Engineering
Building Materials&Construction Engineering
-
 Electronic & Electrical Appliances
Electronic & Electrical Appliances
-
 Toys, Furniture & Home Decoration
Toys, Furniture & Home Decoration
-
 Industrial Equipment & Manufacturing
Industrial Equipment & Manufacturing
-
 Rail & Aviation
Rail & Aviation
-
 Automotive & Spare Parts
Automotive & Spare Parts
-
 Pharma and Medical Services
Pharma and Medical Services
-
 Maritime Vessel Compliance Testing
Maritime Vessel Compliance Testing
 By Industry
By IndustryOur service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
-
 Specialty
SpecialtyComprehensively guarantee quality and safety, promote compliance and innovation, demonstrate brand competitiveness, and achieve higher quality, healthier, safer, and greener sustainable development.
-
 Management
ManagementWe have established a clear governance structure in accordance with listing requirements and national regulations and policies to deal with internal and external challenges and achieve sustainable development.
-
 Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
-
 Talents Policy
Talents PolicyEnsuring the basic rights and benefits of employees;
Providing professional skills training to promote employees’ growth;
Carrying out various kinds of activities to balance employees’ work and life.
-
 RecruitmentWelcome to join NG28 family! We are providing a platform for you to show your talents and achieve your career aspiration.
RecruitmentWelcome to join NG28 family! We are providing a platform for you to show your talents and achieve your career aspiration.

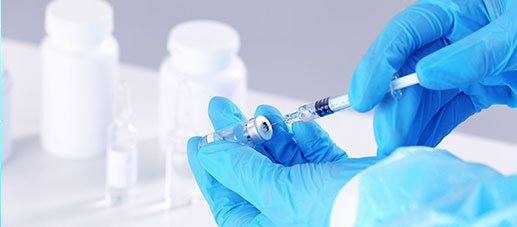
QUALITY & VALUE
NG28 can provide comprehensive product testing and validation services for disposable products (bags, tubes, connectors, filters, etc.) in the biopharmaceutical industry. It can also provide users of disposable products with specific process validation services that meet domestic and international regulatory requirements .

Service Content
|
Extractables and Leachables Testing Extractables and leachables are important indicators for risk assessment of SUS products, NG28 Huatech can conduct extractables and leachables studies on disposable products including membranes, tubing, filters, and other components according to BPOG standardized extraction protocols or USP<665>, <1665>, and establish standardized databases for disposable system manufacturers to meet customers' needs, which can be used to guide the risk assessment of pharmaceutical companies. Risk assessment for pharmaceutical companies. |
Final product quality control SUS final products are usually tested for integrity, visual inspection, cytotoxicity, particulate matter, bacterial endotoxin, RNAase & DNAase, sterility, packaging and transportation confirmation, etc. NG28 can conduct SUS product quality control according to USP<1207>, <87>, <788>, <85>, ISO11737, ISO10993, ASTM D4169 and other standards. D4169 and other standards for SUS end-product quality control, to facilitate rapid compliance and market launch. |
|
Bacterial and Mycoplasma Challenges for Filters NG28 can conduct bacterial retention tests on 0.22μm filters/membranes according to ASTM F838 to verify whether the filters/membranes meet the requirements of sterilizing grade filters. |
One-time system compatibility verification The most prominent issue with the use of SUS products is the potential for compounds to leach from the contact surfaces of the SUS components and enter the process, thus negatively impacting the quality and safety of the drug product. Pharmaceutical regulatory agencies in various countries have set basic requirements for extractables and leachables from SUS. |
|
Sterile Filtration Process Validation Sterilization and filtration is a process that removes microorganisms from a fluid and should not adversely affect product quality. Sterilization filtration validation should include the performance validation of the sterilization filter itself and the filtration process validation, in which the filtration process validation is to validate the sterilization filtration process with respect to the specific fluid and the specific process conditions of the pharmaceutical manufacturing enterprise to ensure that the sterilization filtration process operates reliably under the predefined process conditions. |
Virus Removal Validation for Virus Removal Filters The purpose of the virus removal validation is to assess the ability of the manufacturing process to remove and/or inactivate known viruses. An appropriate amount of indicator virus is added to the sample, a scaled-down model is used to simulate the treatment of the sample with the process parameters, and then the virus titer in the sample before and after treatment is detected, and the virus removal effect of the production process is judged based on the titer reduction value (LRV). |
|
Radiation dose verification NG28 can carry out a full set of irradiation dose validation for irradiated sterilized SUS products according to the requirements of ISO11137 standard, and complete the regular audit of VDmax irradiation dose of SUS products according to ISO11737-1 and ISO11737-2. |
- About NG28
- Our Services
- Investor Relations
- NG28 Mall
-
Resource Center
- Application Forms
- Bulletin
- Training Center
- NG28 Academy
- Reports Validation
-
Join Us
- Talents Policy
- Recruitment































 粤公网安备 44030602000441号
粤公网安备 44030602000441号 