
QUALITY & VALUE
Refers to the medical equipment's only risk reducing measure failing or exhibiting one form of abnormality. Under the state of this single fault, the medical electrical equipment still needs to maintain product safety.
- Consulting quotation
- Online shopping mall
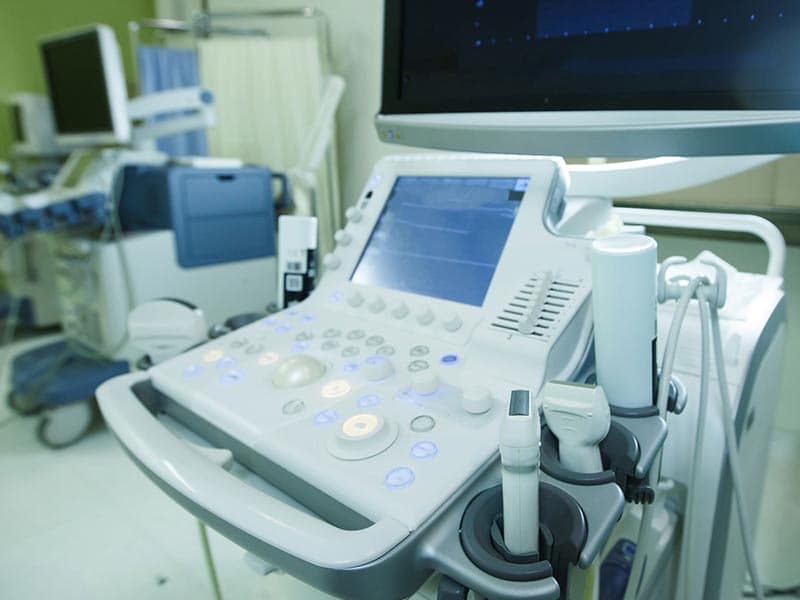
? Service Background
Single Fault:Refers to the medical equipment's only risk reducing measure failing or exhibiting one form of abnormality. Under the state of this single fault, the medical electrical equipment still needs to maintain product safety. The Single Fault test, which evaluates quality and electrical safety characteristics, is one of the most important tests for safety of medical electrical products
Medical device testing is the basis for medical devices to enter the global market. If a product is to be exported to the European Union or the United States, then corresponding tests will need to be done to meet the EU or US standards.
? Other Services of Medical Devices
- Testing of active medical device
- EMC testing and debugging service
- Reliability Testing
- NMPA registration testing service
- Validation of product life
- Failure mode analysis
- Biocompatibility test
- Software evaluation
- Global marketing access
- US agent service
- EU representative service
- Training Service
? Medical Device Testing Standards
In Vitro Diagnostic Medical Devices
|
SN |
Title |
International Standard |
National Standard |
|
1 |
Electrical Equipment For Measurement, Control, and Laboratory Use |
IEC/EN 61010-1 |
GB4793.1 |
|
2 |
Safety requirements for electrical equipment for measurement, control and laboratory use –Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical equipment |
IEC/EN 61010-2-101 |
YY 0648 |
|
3 |
Safety requirements for electrical equipment for measurement, control and laboratory use – Part 081: Particular requirements for automatic and semiautomatic laboratory equipment for analysis and other purposes |
IEC/EN 61010-2-081 |
GB4793.9 |
|
4 |
Electrical equipment for measurement, control and laboratory use – EMC requirements - Part 1: General requirements |
IEC/EN 61326-1 |
GB/T 18268.1 |
|
5 |
Electrical equipment for measurement, control and laboratory use – EMC requirements - Part 2-6: Particular requirements - In vitro diagnostic (IVD) medical equipment |
IEC/EN 61326-2-6 |
GB/T18268.26 |
Medical Device Testing Standards List
|
SN |
Title |
International Standard |
National Standard |
|
1 |
Medical safety and electrical essential equipment performance - Part 2-25: of electrocardiographs Particular requirements |
IEC/EN 60601-2-25 |
GB 10793 |
|
2 |
Non-invasive sphygmomanometers - Part 1: Requirements and test methods for non-automated measurement type |
EN ISO /ISO 81060-1 |
|
|
3 |
Non-invasive sphygmomanometers - Part 3: Supplementary requirements for electro-mechanical blood pressure measuring systems |
EN 1060-3 |
|
|
4 |
Medical electrical equipment -- Part 1-1: General requirements for safety - Collateral standard: Safety requirements for medical electrical systems |
IEC/EN 60601-1-1 |
GB 9706.15 |
|
5 |
Medical electrical equipment - Part 2-27: Particular requirements for the basic safety and essential performance of electrocardiographic monitoring equipment |
IEC/EN 60601-2-27 |
GB 9706.25 |
|
7 |
Medical electrical equipment-Part 2-26: Particular requirements for the safety of electroencephalographs |
IEC/EN 60601-2-26:2012 IEC 80601-2-26:2019 |
GB 9706.26 |
|
8 |
Medical safety and electrical essential equipment-Part performance2-56: of clinical Particular thermometers requirements for basic body temperature measurement |
ISO/ EN ISO 80601-2-56 |
|
|
9 |
Non-invasive automated sphygmomanometers |
ANSI/AAMI SP10 |
YY 0670 |
|
10 |
Medical electrical equipment - Part 2-57: Particular requirements for the basic safety and essential performance of non-laser light source equipment intended for therapeutic, diagnostic, monitoring and cosmetic/aesthetic use |
IEC/EN 60601-2-57 |
|
|
11 |
Medical Basic Safety Electrical And Equipment Essential -Performance Part 2-2: Particular Of High Requirements Frequency For Surgical Equipment And High Frequency Surgical Accessories |
IEC/EN/EN IEC 60601-2-2 |
GB 9706.4 |
|
12 |
Medical electrical equipment - Part 1: General requirements for basic safety and essential performance |
ANSI/AAMI ES60601-1 EN /IEC60601-1 |
GB9706.1 |
|
13 |
Medical electrical equipment - Part 2-10 : particular requirements for the safety of nerve and muscle stimulators |
IECC/EN 60601-2-10 |
YY 0607 |
|
14 |
Medical electrical equipment - Part 2-30: Particular requirements for basic safety and essential performance of automated non-invasive sphygmomanometers |
IEC/EN 80601-2-30 |
YY 0667 |
|
15 |
Medical electrical equipment-Part 2-49: Particular requirements for the safety of multifunction patient monitoring equipment |
IEC/EN 60601-2-49/IEC 80601-2-49 |
|
- About NG28
- Our Services
- Investor Relations
- NG28 Mall
-
Resource Center
- Application Forms
- Bulletin
- Training Center
- NG28 Academy
- Reports Validation
-
Join Us
- Talents Policy
- Recruitment





































 粤公网安备 44030602000441号
粤公网安备 44030602000441号 